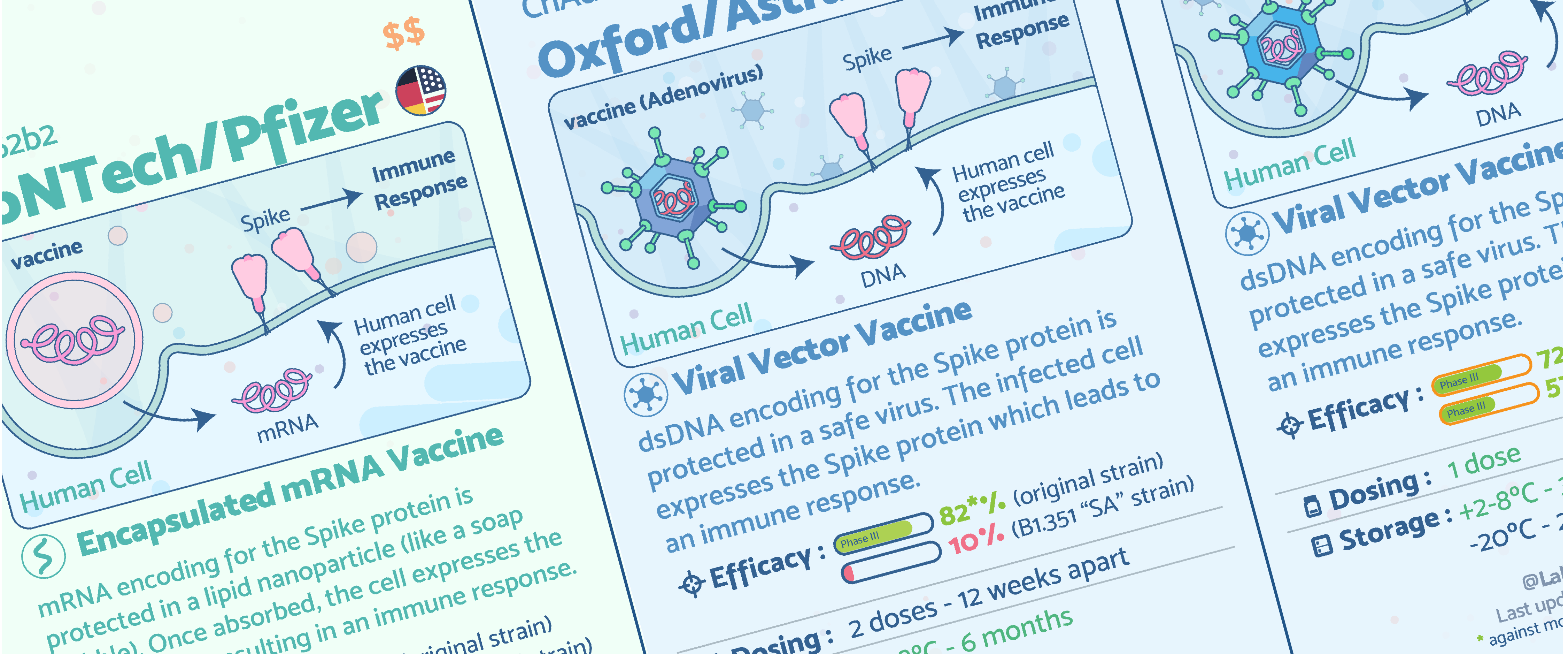
COVID-19 vaccine cards
Flashcard cards about each approved vaccine
4 March 2021

Flashcard cards about each approved vaccine
4 March 2021

How is it made and is it scary?
10 January 2018
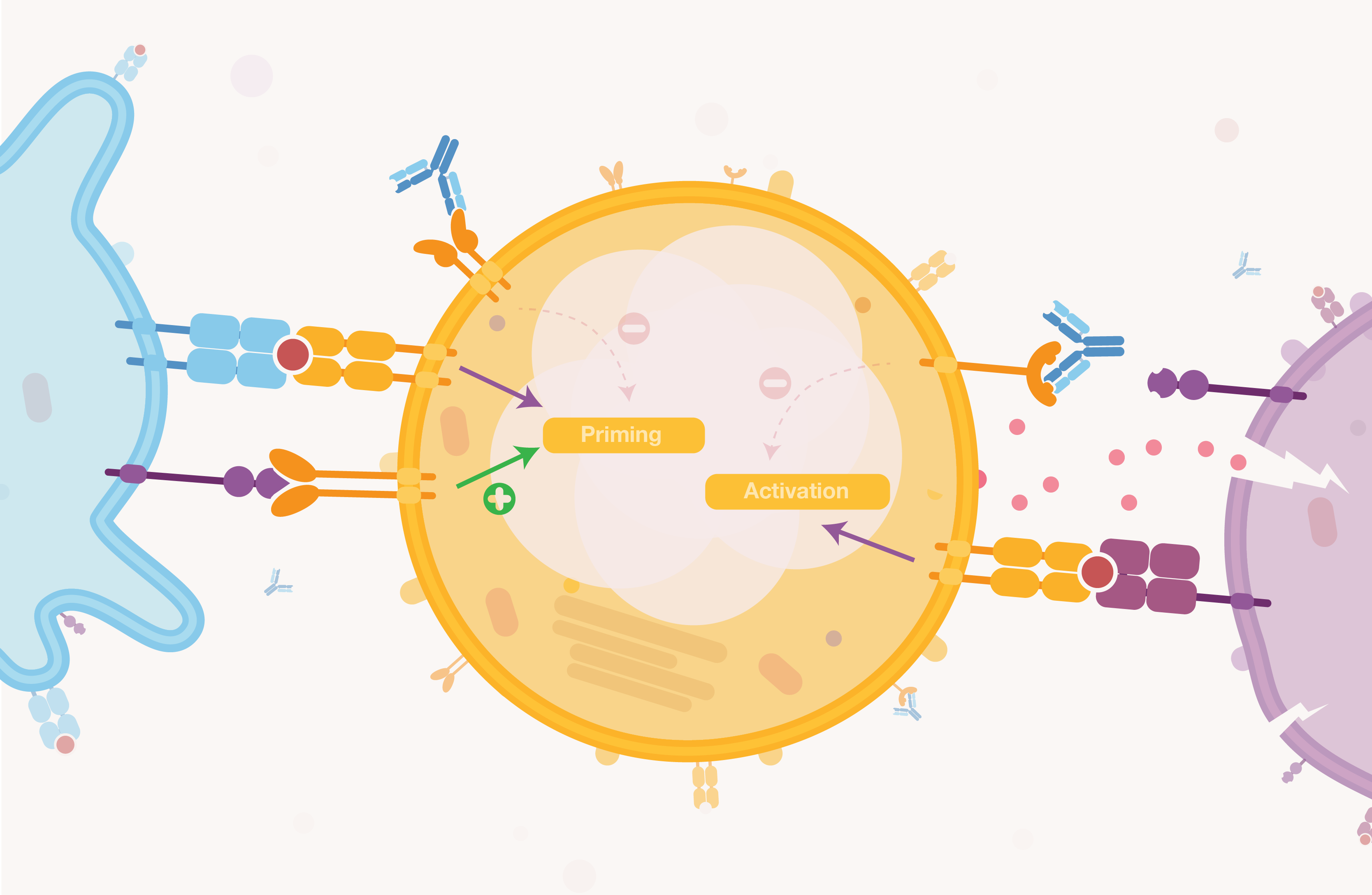
how do they work and why are they cool?
10 January 2018
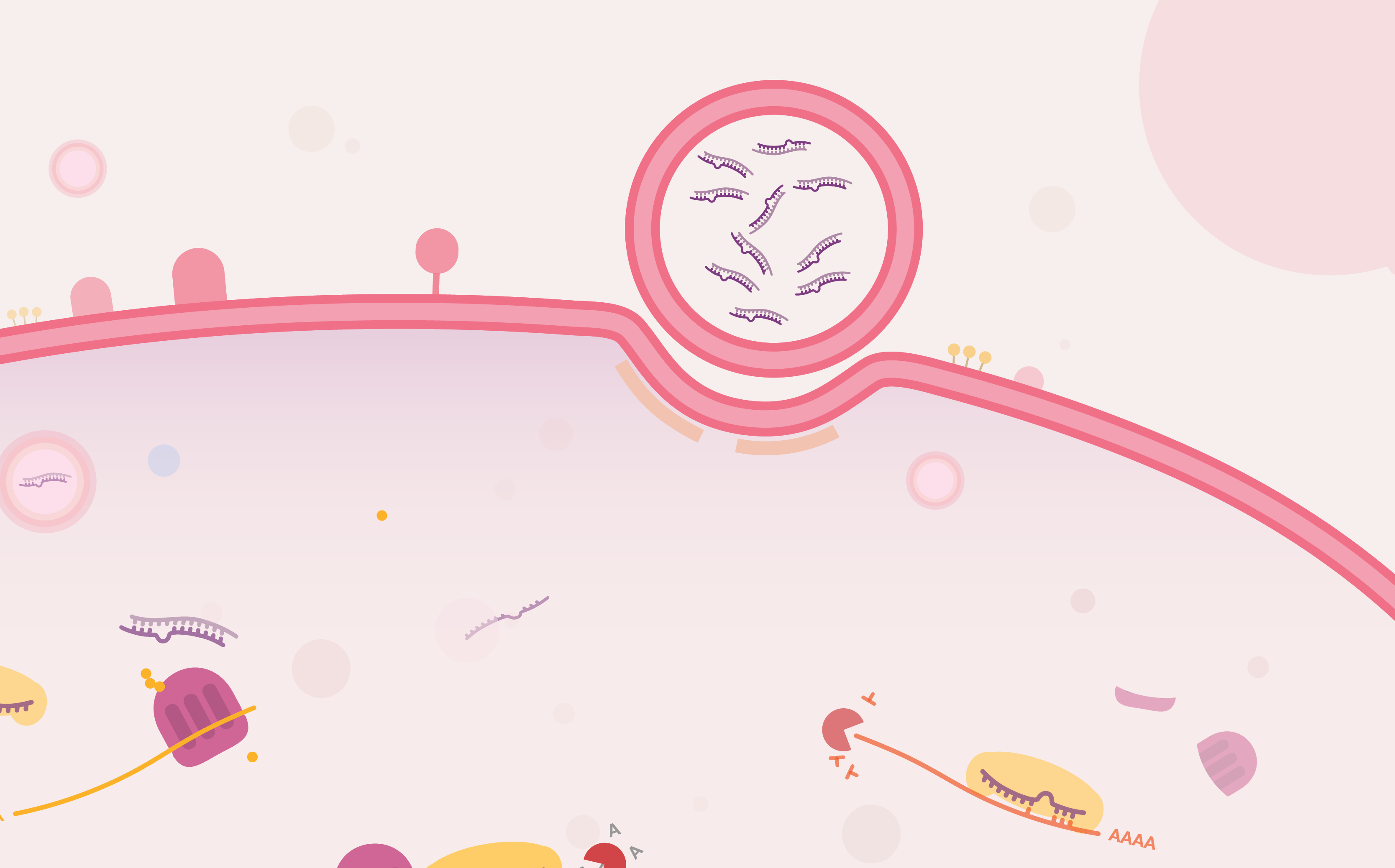
Targeting the Untargetable
15 November 2017
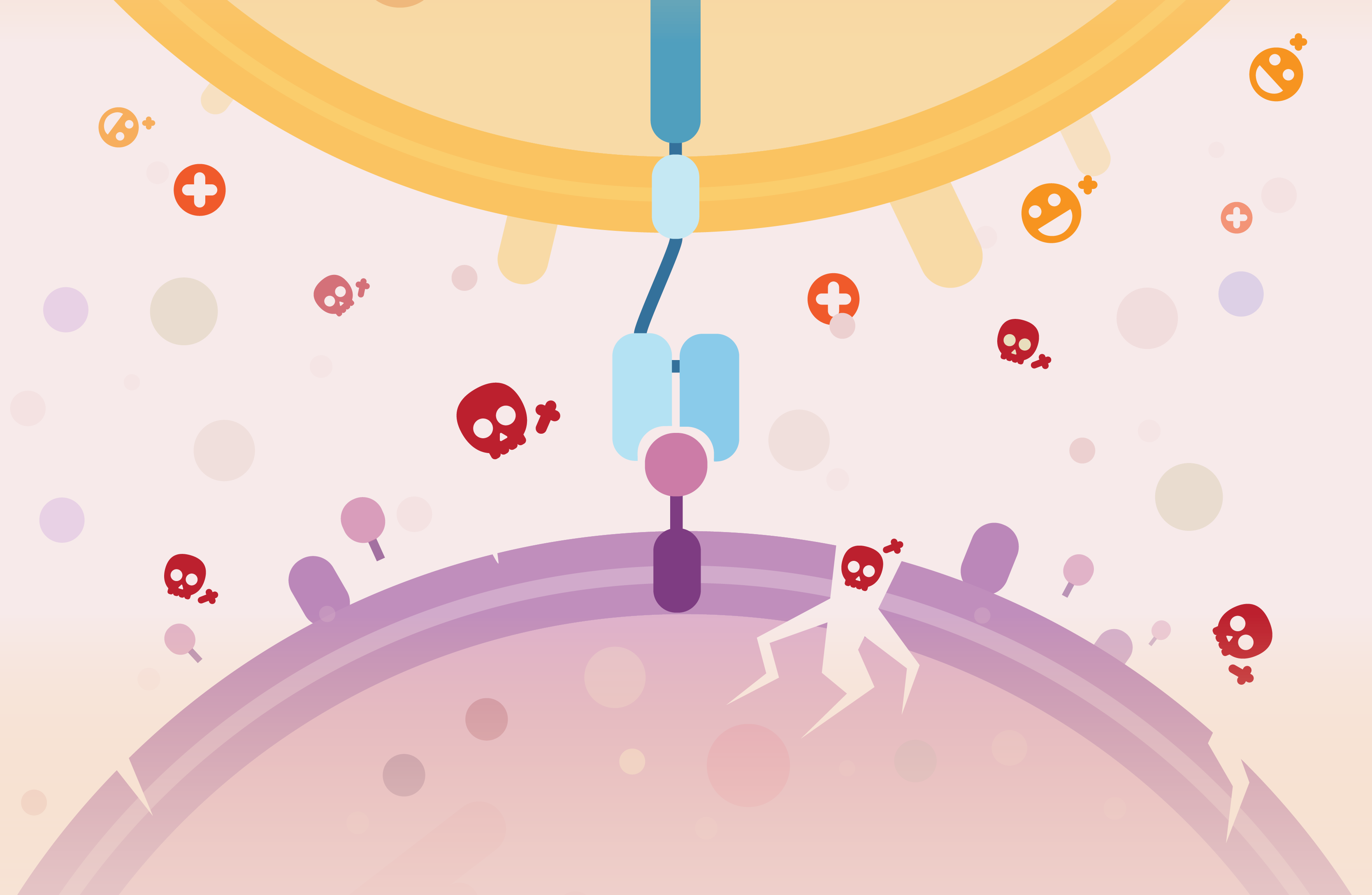
How do they fight cancer?
12 October 2017

About ribosomes and mRNA
10 January 2016








English ‘V2’
last updated in May 2021
Download the cards as .zip
Download the cards as .pdf
English ‘V1’
last updated in March 2021
Download the cards as .zip
French - Français ‘V1’ translated by LaPipette!
last updated in March 2021
Download the cards as .zip
Simplified Chinese - 简体中文 ‘V1’ translated by Chenlu Yu
last updated in March 2021
Download the cards as .zip
Traditional Chinese - 繁體中文 ‘V1’ translated by Irene Yeung and Hau Kwan Abby Lo
last updated in March 2021
Download the cards as .zip
Traditional Chinese (Cantonese) - 繁體中文(廣東話) ‘V1’ translated by Hau Kwan Abby Lo and Irene Yeung
last updated in March 2021
Download the cards as .zip
Spanish - Español ‘V1’ translated by BioPosts
last updated in March 2021
Download the cards as .zip
Portuguese - Português (Brasil) ‘V1’ translated by Julio Cesar Lorenzi
last updated in March 2021
Download the cards as .zip
Italian - Italiano ‘V1’ translated by Renzo Toffolo
last updated in March 2021
Download the cards as .zip
Slovak - Slovensky ‘V1’ translated by Patrícia Hrašnová
last updated in March 2021
Download the cards as .zip
Serbian - Srpski ‘V1’ translated by Ivana Prokić from Ujedinjeni Protiv Kovida
last updated in March 2021
Download the cards as .zip
Serbian - Српски ‘V1’ translated by Miloš Vasić
last updated in March 2021
Download the cards as .zip
Czech - čeština ‘V1’ translated by Julius Luke
last updated in March 2021
Download the cards as .zip
Romanian - Română ‘V1’ translated by Razvan Nastasa
last updated in March 2021
Download the cards as .zip
Indonesian - Bahasa Indonesia ‘V1’ translated by Adien Esti
last updated in March 2021
Download the cards as .zip
Vietnamese - Tiếng Việt ‘V1’ translated by Ly Nguyen
Download the cards as .zip
Finnish - Suomalainen ‘V1’ translated by Jani Räty
last updated in March 2021
Download the cards as .zip
Georgian - ქართული ‘V1’ translated by Giorgi Chkheidze
last updated in March 2021
Download the cards as .zip
Korean - 한국어 ‘V1’ translated by Jisu Im
last updated in March 2021
Download the cards as .zip
Russian - русский ‘V1’ translated by Kristina Sagaliec
last updated in March 2021
Download the cards as .zip
German - Deutsch ‘V1’ translated by Doğan Doruk Demircioğlu
last updated in March 2021
Download the cards as .zip
Japanese - 日本語 ‘V1’ translated by Yo Nakahara
last updated in March 2021
Download the cards as .zip
Hungarian - Magyar ‘V1’ translated by Attila Imre
last updated in March 2021
Download the cards as .zip
Dutch - Nederlands ‘V1’ translated by Iris Iemenschot
last updated in March 2021
Download the cards as .zip
Bosnian - Bosanski ‘V1’ translated by Zerina Kurtovic
last updated in March 2021
Download the cards as .zip
Albanian - Shqiptare ‘V1’ translated by Orion JUCJA
last updated in March 2021
Download the cards as .zip
Polish - Polskie ‘V1’ translated by Marzena Ciechomska from PSNC as part of the REGIONAL COVID-HUB
last updated in March 2021
Download the cards as .zip
Ukrainian - Український ‘V1’ translated by Anastasiia Filimonova
last updated in March 2021
Download the cards as .zip
Macedonian - Македонски ‘V1’ translated by Dimitar Stevchev
last updated in March 2021
Download the cards as .zip

Thanks for reading! Let us know what you think!

2018-01-10 00:00:00 +0000
A cultured burger is made of billion of muscle strands which were grown in Petri dishes in the lab using cell culture techniques. Scientists are now able to regenerate tissues from stem cells and the same principles can also be used to produce meat! Right now the few cells needed are collected by a small biopsy which does not injure the animal but similarly to traditional cell culture techniques we could envision that scientists will generate stable cell lines in order to grow meat thus removing the need for any animal sample. The biggest issue to tackle is actually the scaling up of the process in order to lower the costs of the final meat.
This article focuses mainly on beef but chicken, duck and even tuna will reach the market probably in the next 5 years. This new type of meat could allow us to consume meat without the need for animals! Is it safe and is this the future of meat? To learn more about cultured meat check the infographic below!
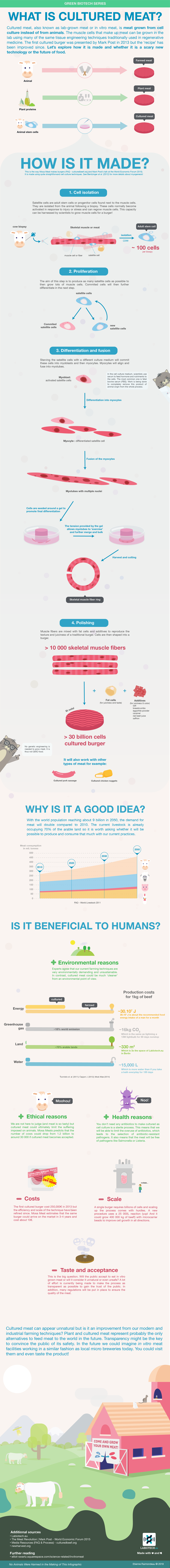
Thanks for reading! Let us know what you think!

2018-01-10 00:00:00 +0000
In 2013, Jimmy Carter's miracle remission of cancer was achieved thanks to a new type of therapy: checkpoints inhibitors. Checkpoints are signals used by cells to modulate the immune response either by stimulating it or inhibiting it. Cancer cells hack this pathway and express molecules activating inhibitory checkpoints. This silences the immune response, hence making the cancer cell "invisible" to the immune cells. Checkpoints inhibitors prevent this escaping mechanism. This is great because therapies using checkpoint inhibitors could be used in combination with more traditional anti-cancer approaches.
The potential of checkpoints was only discovered a few years ago but these types of molecules can boast more than 1500 clinical trials (in 2017 - time of writing) going on currently (which is an insane number). It is estimated that by 2025, the market for inhibitors could reach near €30Bn!
Let’s explore what checkpoints inhibitors are and why they are a very popular new type of therapy against cancers
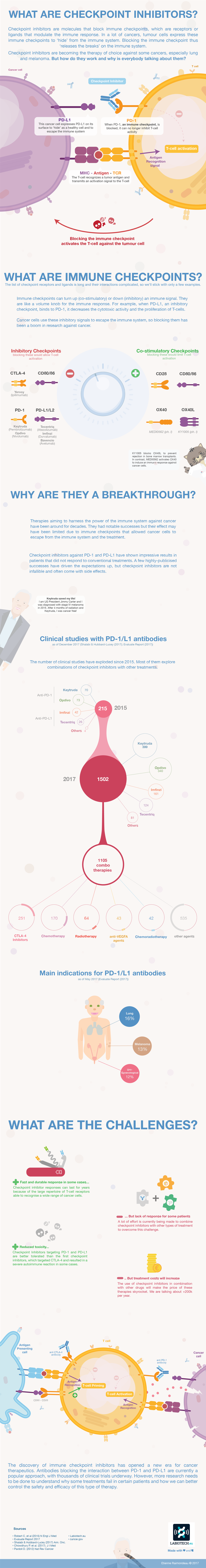
Thanks for reading! Let us know what you think!

2017-11-15 00:00:00 +0000
Viruses have long exploited the expression machinery of cells to translate their genome into proteins. In response, defence mechanisms arise in different types of cells. In one of them called RNA interference, the infected cell produces a short RNA that target and destroy the foreign RNA. Cells have since used this mechanism to also regulate their own RNA level and thus protein level. This mechanism which was discovered 20 years ago can be hacked by scientists to target specific mRNA responsible for diseases.
By feeding cells specific short RNAs, scientists can virtually target any expressed gene. For example, silencing abnormally expressed genes in cancer cells is possible with RNAi. However, RNAs are very hard to deliver to a cell but as the technology progress, RNAi therapies are getting closer to the market.
Everything you need to know about RNAi therapies is packed into this infographic:
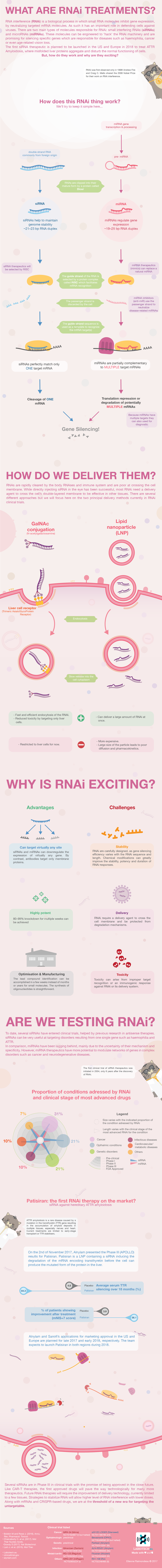
Thanks for reading! Let us know what you think!

2017-10-12 00:00:00 +0000
The idea of immunotherapy or using our own immune system against cancer has been around for more than 60 years. At the time, the absence of techniques for culturing and manipulating cells made it impossible to harness the power of immune cells. Decades later, scientists were able to design and produce antibodies targeting specific molecules on the surface of cancer cells. Such therapies were only approved for patients in the late 90s.
More recently, a radical new approach has been allowed by genome editing enzymes such as TALENs and the famous CRISPR proteins : to engineer immune cells to target cancer. Lymphocytes are the specialised soldiers in the immune system army, in particular, lymphocytes T (T cells) are able to find and kill abnormal cells as well as coordinate attacks by the other immune cells. They thus constitute a perfect candidate for scientists to engineer an immune response against cancer. Antigen receptors are ‘molecular scanners’ at the surface of the T cells enabling the detection of abnormal cells. These can be modified via genome editing to force T cells to bind and destroy cancer cells. This new generation of immunotherapies has been showing incredible positive results during clinical trials which led to the approval of the first CAR-T few months ago.
Let’s explore what CAR-T are, how we make them: and why this is an exciting new way to fight cancer:
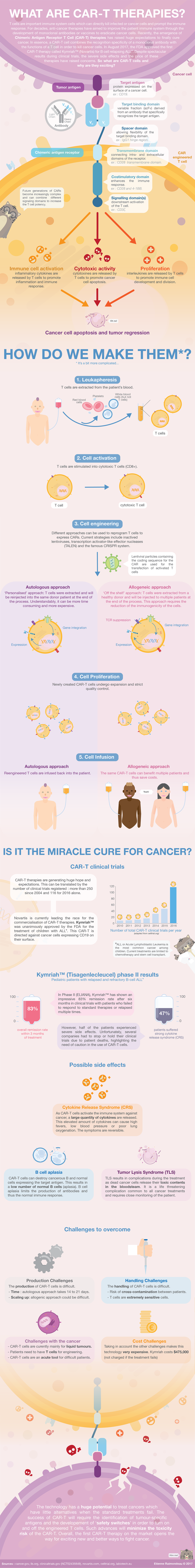
Thanks for reading! Let us know what you think!

2016-01-10 00:00:00 +0000
This project gathers some of the illustrations I created for my PhD thesis manuscript. The whole document was written in LaTeX using Lyx. Illustrations were done with Illustrator.



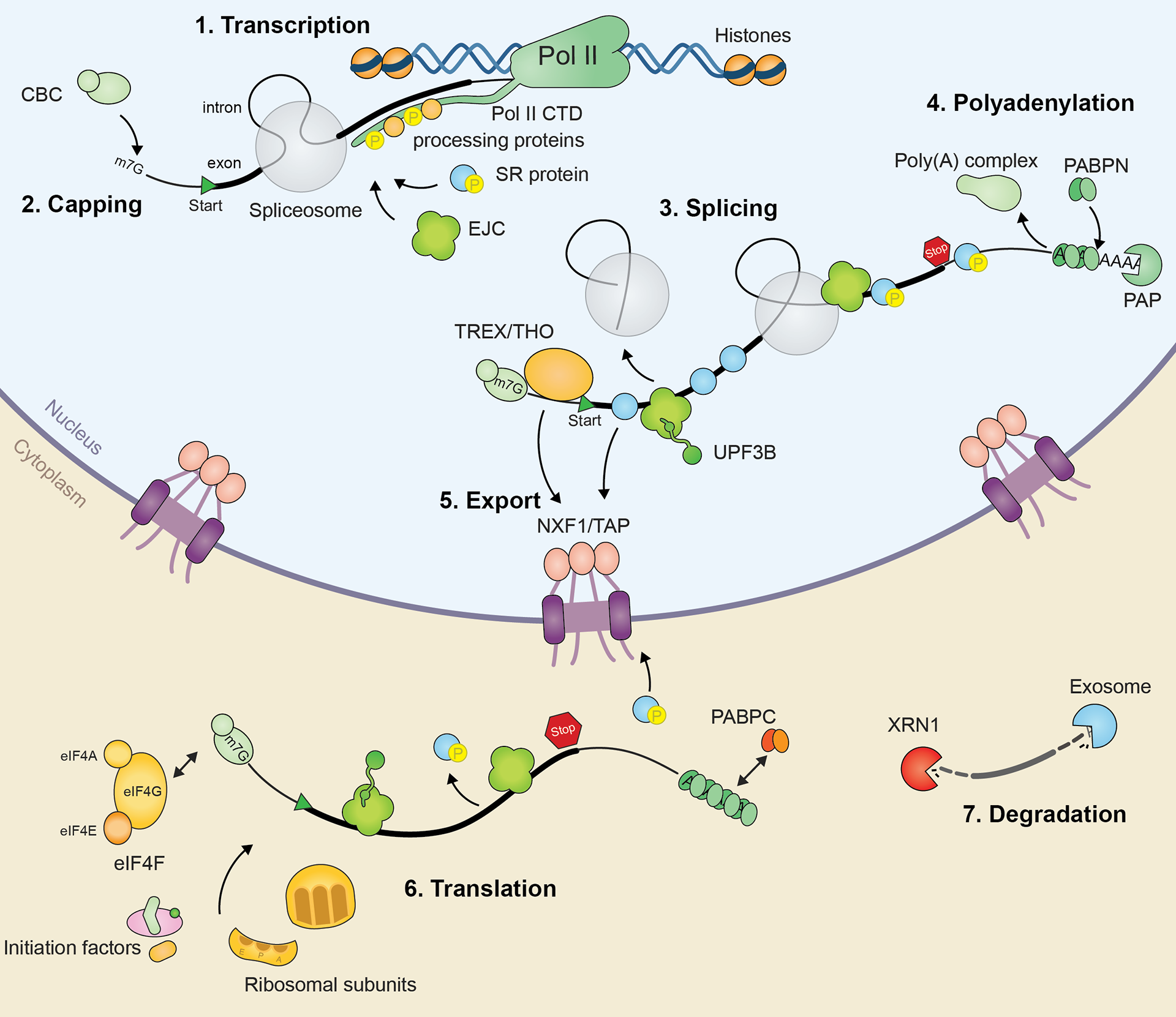
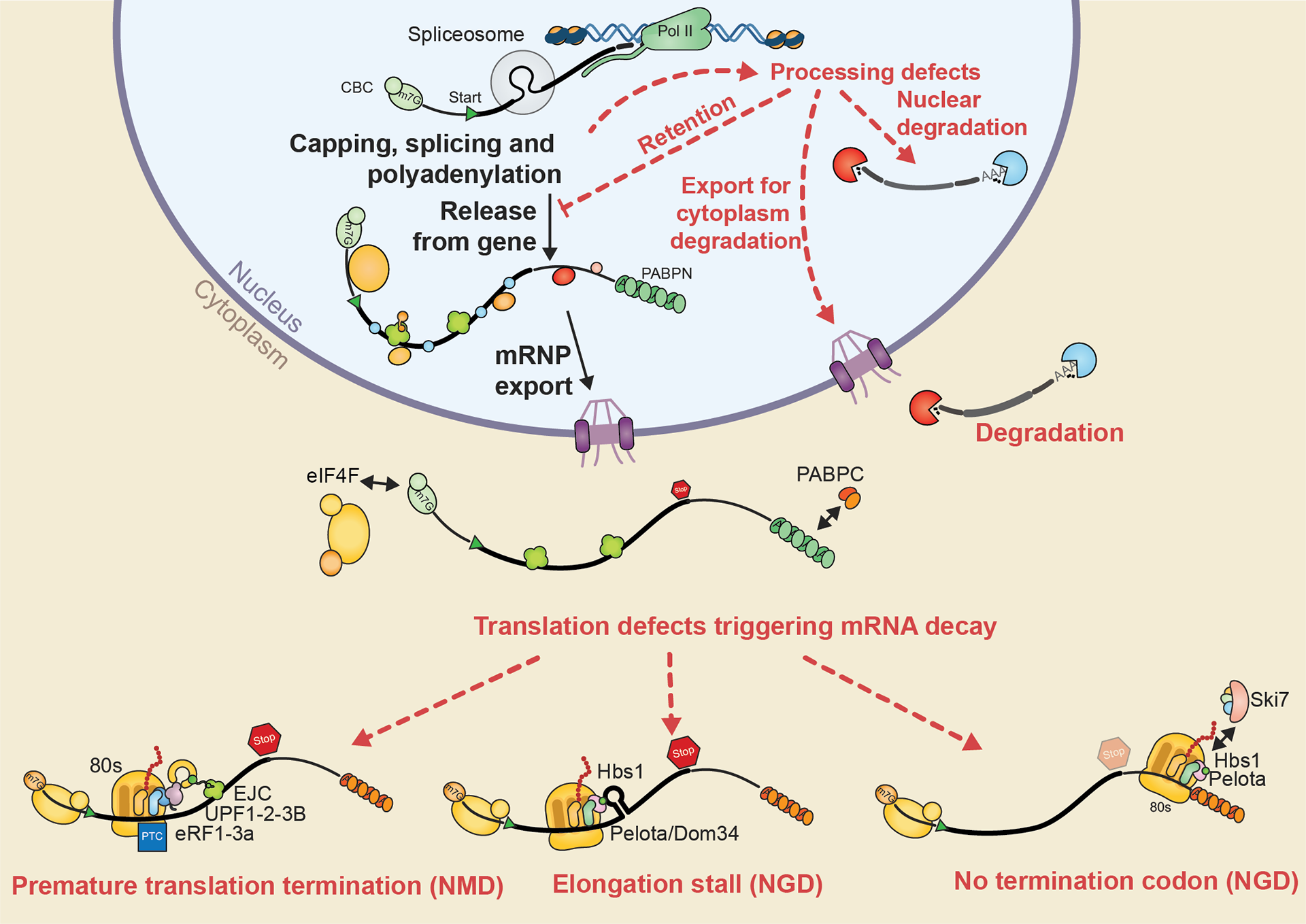
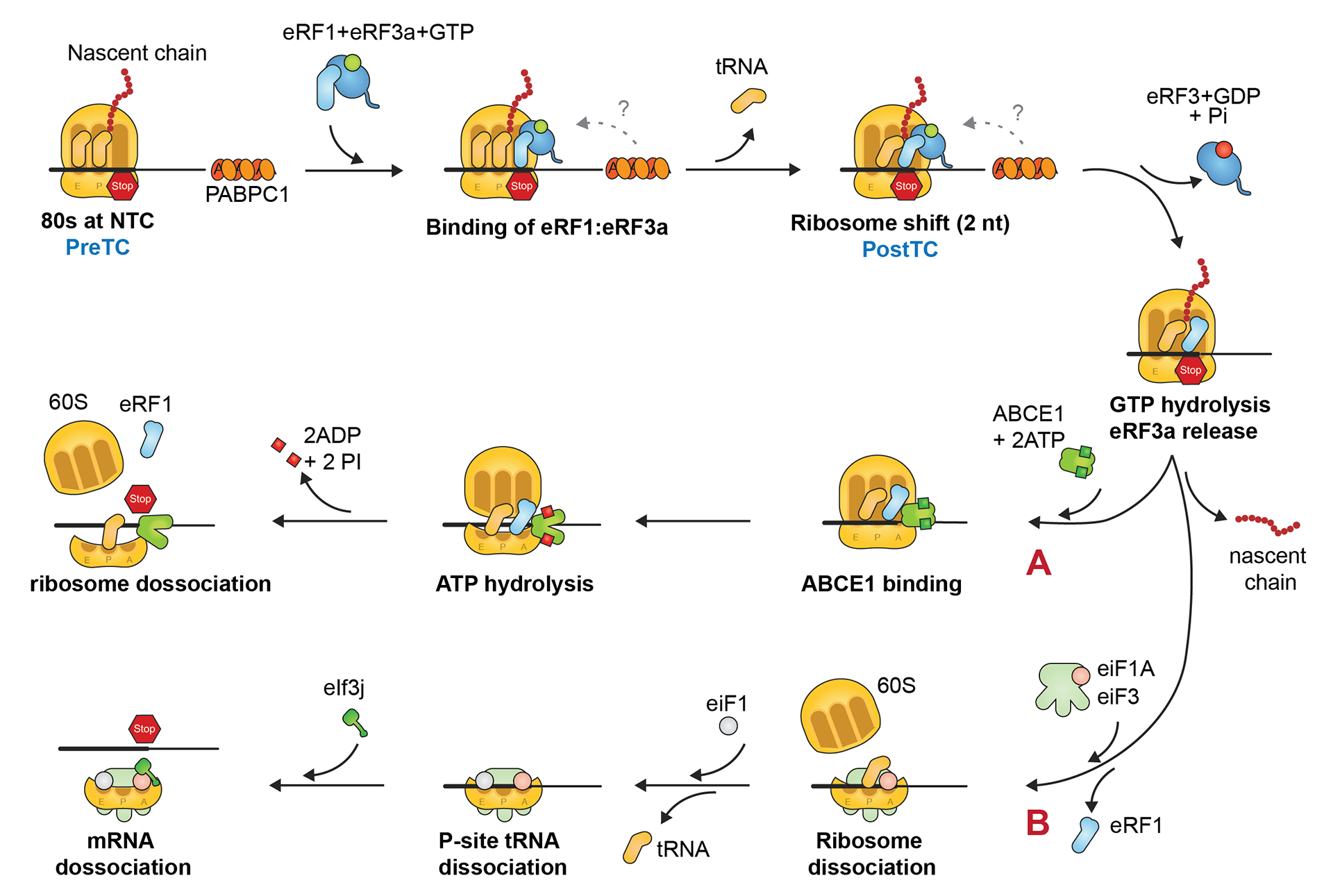
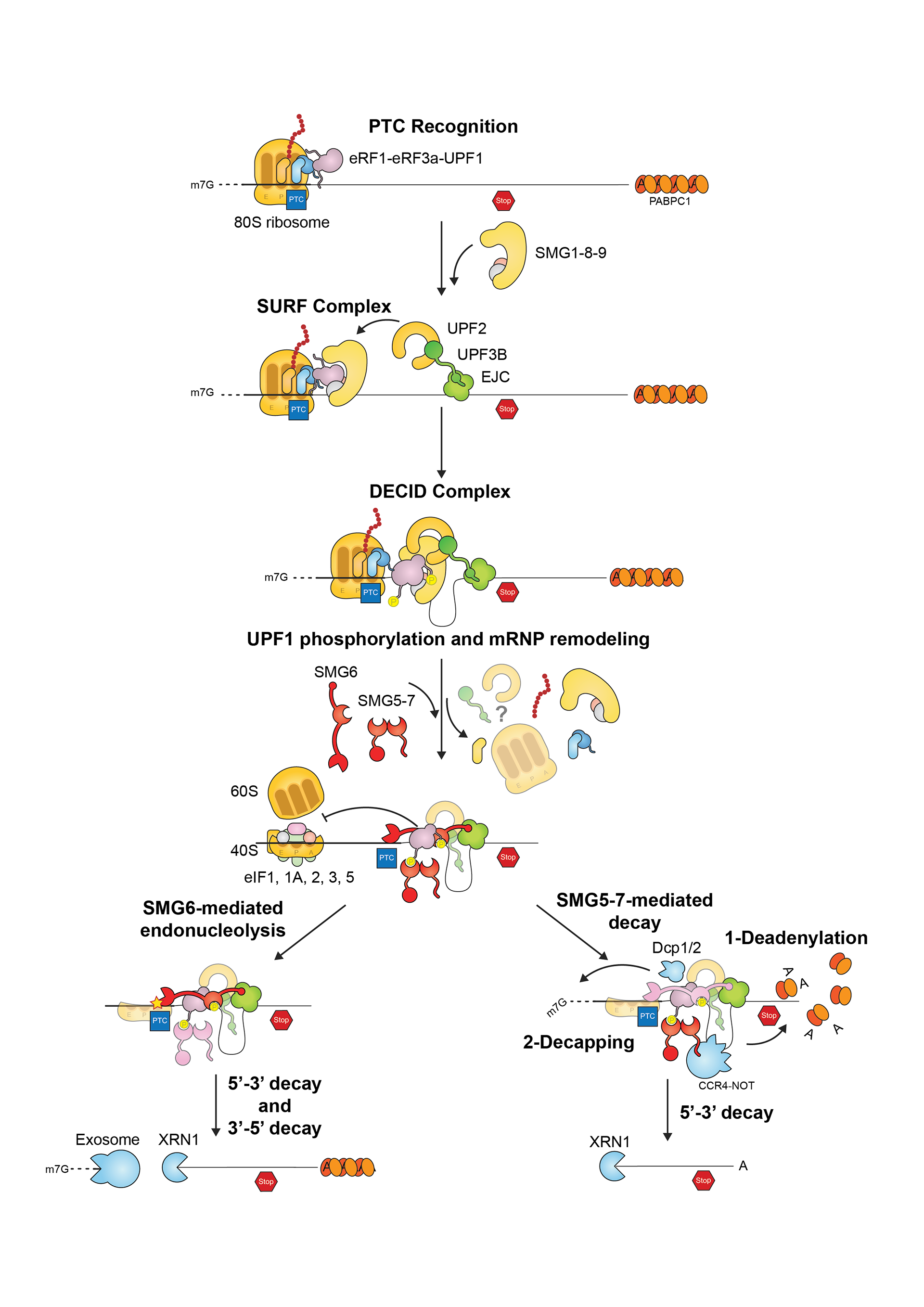
Thanks for reading! Let us know what you think!