Targeting the Untargetable
Context
Viruses have long exploited the expression machinery of cells to translate their genome into proteins. In response, defence mechanisms arise in different types of cells. In one of them called RNA interference, the infected cell produces a short RNA that target and destroy the foreign RNA. Cells have since used this mechanism to also regulate their own RNA level and thus protein level. This mechanism which was discovered 20 years ago can be hacked by scientists to target specific mRNA responsible for diseases.
RNA interference or RNAi therapies have been imagined decades ago. However, they will only arrive on the market in 2018. Why are RNAi therapies exciting and why the wait was so long?
By feeding cells specific short RNAs, scientists can virtually target any expressed gene. For example, silencing abnormally expressed genes in cancer cells is possible with RNAi. However, RNAs are very hard to deliver to a cell but as the technology progress, RNAi therapies are getting closer to the market.
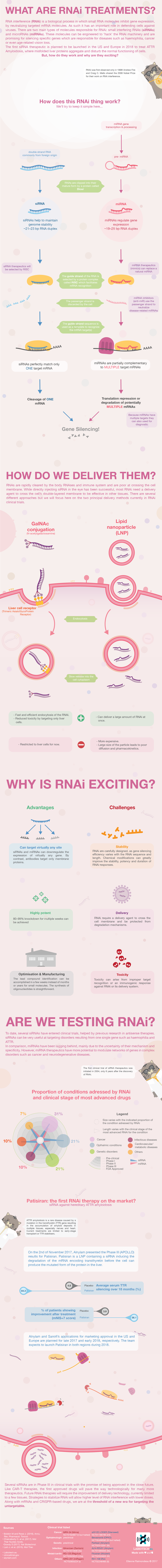
Everything you need to know about RNAi therapies is packed into this infographic:
References
Science Articles
- Bobbin M and Rossi J. (2016). RNA Interference (RNAi)-Based Therapeutics: Delivering on the Promise? Annu. Rev. Pharmacol. Toxicol.
- Chakraborty C. et al. (2017). Therapeutic miRNA and siRNA: Moving from Bench to Clinic as Next Generation Medicine. Molecular Therapy: Nucleic Acids
- Dowdy S. (2017). Overcoming cellular barriers for RNA therapeutics. Nature Biotechnology
- Lam J. et al. (2015). siRNA Versus miRNA as Therapeutics for Gene Silencing. Molecular Therapy—Nucleic Acids Vol {:target=”_blank”}
Web
- Labiotech.eu, “RNA as a Therapy: Reviewing the Future Generation of Therapeutics”
- Labiotech.eu, “Ultimate Review: How Could mRNA Overtake all other Biologicals in Medicine?”
- Alynam - “Our pipeline”
Clinical trial listed
- Cancer
- miRNA mimic: MRX-34 (Mirna) - Phase I (alted) - NCT01829971
- siRNA: siG12D LODER (Silenseed) - Phase I/II completed - NCT01188785
- Ophtalmologic:
- miRNA: preclinical
- siRNA: Bevasiranib (OPKO) - Phase III abandoned - NCT00499590
- Genetic:
- miRNA: miRNA inhib: RG-012 (Regulus/Sanofi) - Phase II - NCT02855268
- siRNA: Partisan (Alnylam) - Phase III NCT01960348 & NCT02510261
- Infection:
- miRNA inhib: miravirsen (Santaris) - Phase IIa - NCT01872936
- siRNA: ALN-RSV01 (Alnylam) - Phase IIb completed - NCT01065935
- Metab-cardio:
- miRNA inhib: RG-125 (Regulus/Sanofi) - Phase I/IIa - NCT02826525
- siRNA: fitusiran (Alnylam/Sanofi) - Phase III - NCT02035605
- Others:
- miRNA mimics: MRG-201 (miRagen) - Phase I NCT02603224
- siRNA: RX1-109 (RXi Pharma) - Phase II - NCT02246465
Thanks for reading! Let us know what you think!